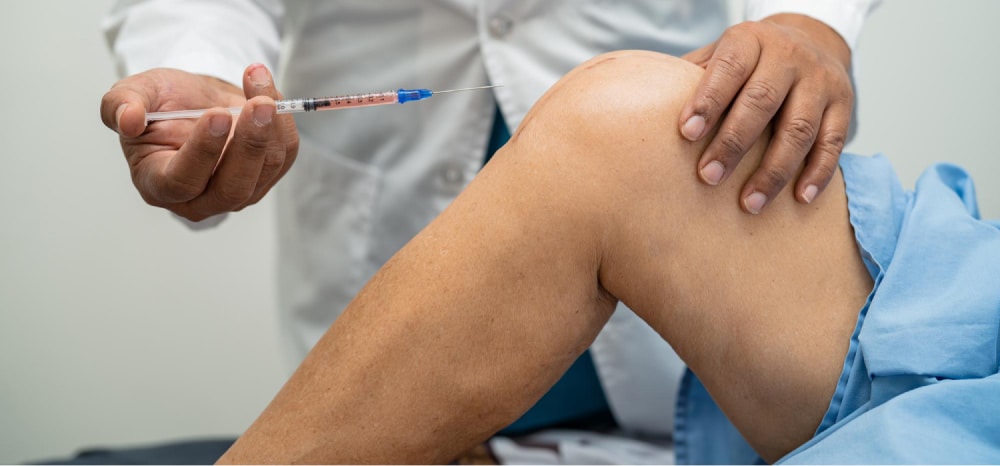
Osteoarthritis (OA) is a prevalent joint disease that leads to the gradual breakdown of cartilage, resulting in pain, inflammation, and diminished joint function. Hyalgan (sodium hyaluronate) injections, commonly known as viscosupplements or hyaluronic acid injections, offer a non-surgical solution to alleviate symptoms and potentially slow the progression of OA. This treatment enhances joint lubrication and cushioning, providing patients with improved mobility and pain relief.
Hyalgan is a viscosupplement composed of hyaluronan, a natural substance found in the synovial fluid of joints. This component is vital for lubricating and cushioning joints, enabling smooth and pain-free movement. Unlike traditional treatments such as NSAIDs and corticosteroids, which primarily address symptoms, Hyalgan targets the underlying issues by replenishing hyaluronan levels in the affected joint.
Clinical studies have demonstrated that Hyalgan injections not only provide symptomatic relief but can also slow the progression of osteoarthritis, making it a valuable therapeutic option for patients with mild to moderate OA.
Not all patients are suitable candidates for Hyalgan injections. Ideal candidates include adults with mild to moderate osteoarthritis who have not responded adequately to conventional therapies. A thorough assessment of the patient’s medical history, disease severity, and previous treatments is essential. Patients with severe OA, joint infections, or skin diseases at the injection site are typically not suitable for Hyalgan therapy.
Administering Hyalgan requires precision and adherence to proper technique:
Aftercare Guidelines
Following Hyalgan injections, patients should:
Benefits:
Potential Side Effects:
Hyalgan injections represent a valuable treatment option for managing osteoarthritis, providing both symptomatic relief and potential disease-modifying effects. Through careful patient selection, proper administration, and vigilant aftercare, Hyalgan can significantly improve joint function and quality of life for those suffering from OA.
Join our newsletter to receive latest news and offers

Medicle MD Ltd
Reg. Number: 14317237
Address: 27 Old Gloucester Street,
WC1N 3AX London,
United Kingdom
Our website is intended solely for people who use medical devices, such as dermal fillers, as professionals. It may contain product advertisements targeted only at such people. To enter Nu Derma Supply, please confirm that you are such a person (e.g. a medical professional, cosmetologist, service technician, etc.).